Cancer is a complex disease and manifests in many different forms. In fact, when speaking about cancer we are probably speaking about thousand different diseases and not one.
The high level of heterogeneity between and across different cancer subtypes requires large amounts of data to study them. Luckily, we do have large data sets today. However, the kind of knowledge we can extract from those data sets depends a lot on where we got the data from. For cancer there are two major classes of data sources: lab-maintained cell lines and biopsies taken directly from a patient’s tumor. Both can provide distinct information as summarized in the following slide.
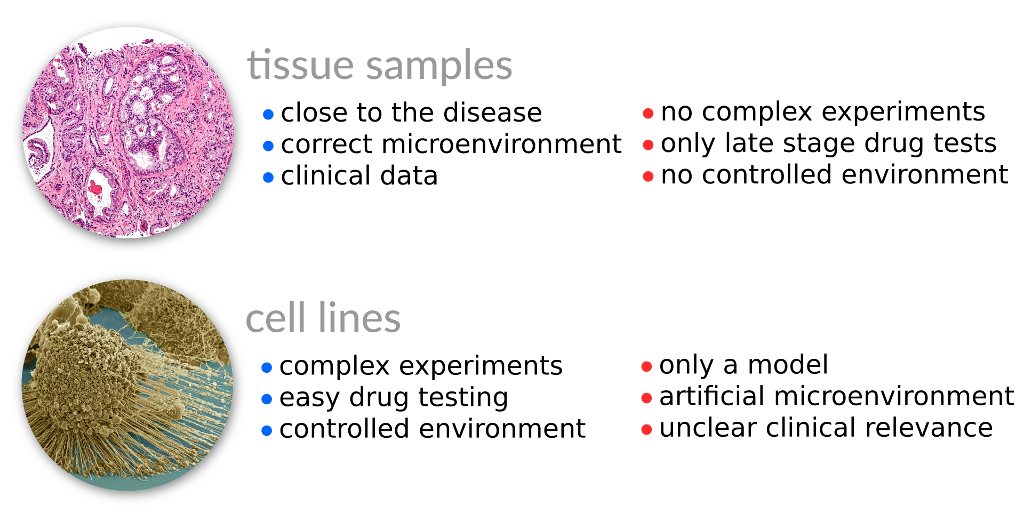
As we can see biopsies may provide large amounts of genomic data, but sometimes lack some phenotype descriptions which are important, for instance the proliferation rate that measures how agressive a tumor multiplies.
In this publication we combined two large cancer data sets, NCI-60 and TCGA. NCI-60 contains genomic data and proliferation rates for 60 cancer cell lines whereas TCGA contains genomic data for cancer biopsies for more than 11.000 patients. By training a a simple model on the NCI-60 data set we predicted proliferation rates for all of 11.000+ tumor samples in TCGA. We observed that proliferation rates may vary substantially even within the same cancer subtype and are associated to patient survival and the tumor stage.
Proliferation rates are also closely connected to the metabolic capacity of a tumor. Thus, we also used metabolic modeling integrating the predicted proliferation rates. This allowed us to predict metabolic alterations that where specific for a distinct cancer subtype. Many of the alterations identified this way have been confirmed by previously published works. In particular, we identified the pentose phosphate pathway, retinol, and branched-chain amino acid metabolism being the most specific alterations.
Even though this study has many limitations, it shows that combining phenotypic and genomic data sets using Machine Learning techniques may provide another level of information and allows for patient-specific predicions of the tumor’s aggresiveness and the metabolic alterations that maintain it.